9th January 2026
Published a paper in FEBS Journal.
Masa Janosev, Tomas Obsil& Veronika Obsilova*. Lock, Relax, Load and Shoot: A Molecular Perspective on Nedd4 Regulation. FEBS Journal (2026). DOI: 10.1111/febs.70424. IF = 4.2
Link: FEBS J. 2026
24th June 2025
Klára Kohoutová defended her PhD thesis.
6th June 2025
Martin Hýbl defended his master thesis (M.Sc.).
26th May 2025
Published a paper in Nature Communications.
Masa Janosev, Dalibor Kosek, Andrej Tekel, Rohit Joshi, Karolina Honzejkova, Pavel Pohl, Tomas Obsil*& Veronika Obsilova*. Structural basis of ubiquitin ligase Nedd4-2 autoinhibition and regulation by calcium and 14-3-3 proteins. Nature Communications 16:4875 (2025). DOI: 10.1038/s41467-025-60207-4. IF = 14.7
* shared corresponding authorship
Link: Nat. Comm. 2025, 16, 4875.
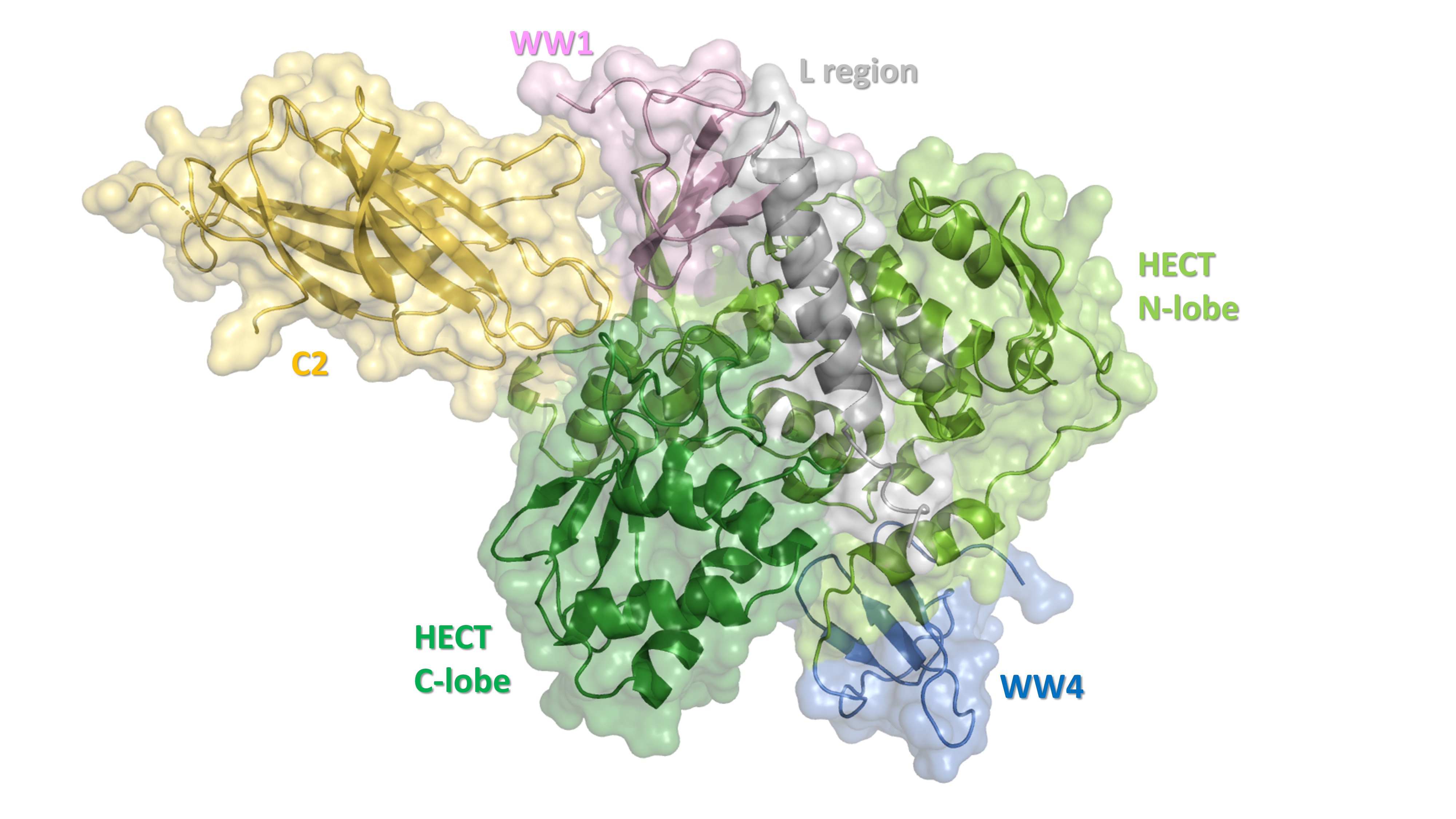
Structure of Nedd4-2 obtained by cryo-EM reconstruction: C2, calcium binding domain (peach); WW1 (pale purple); L region (gray); WW4 (blue); HECT N-lobe, N-lobe of the catalytic HECT domain (light green); HECT C-lobe, C-lobe of the catalytic HECT domain (dark green).
27th May 2025
Published a paper in Nature Communications.
Klara Kohoutova, Pavel Srb, Veronika Obsilova*, Vaclav Veverka* & Tomas Obsil*. Structural plasticity of the FOXO-DBD:p53-TAD interaction. Nature Communications 16:4907 (2025). DOI: 10.1038/s41467-025-59106-5. IF = 14.7
* shared corresponding authorship
Link: Nat. Comm. 2025, 16, 4907

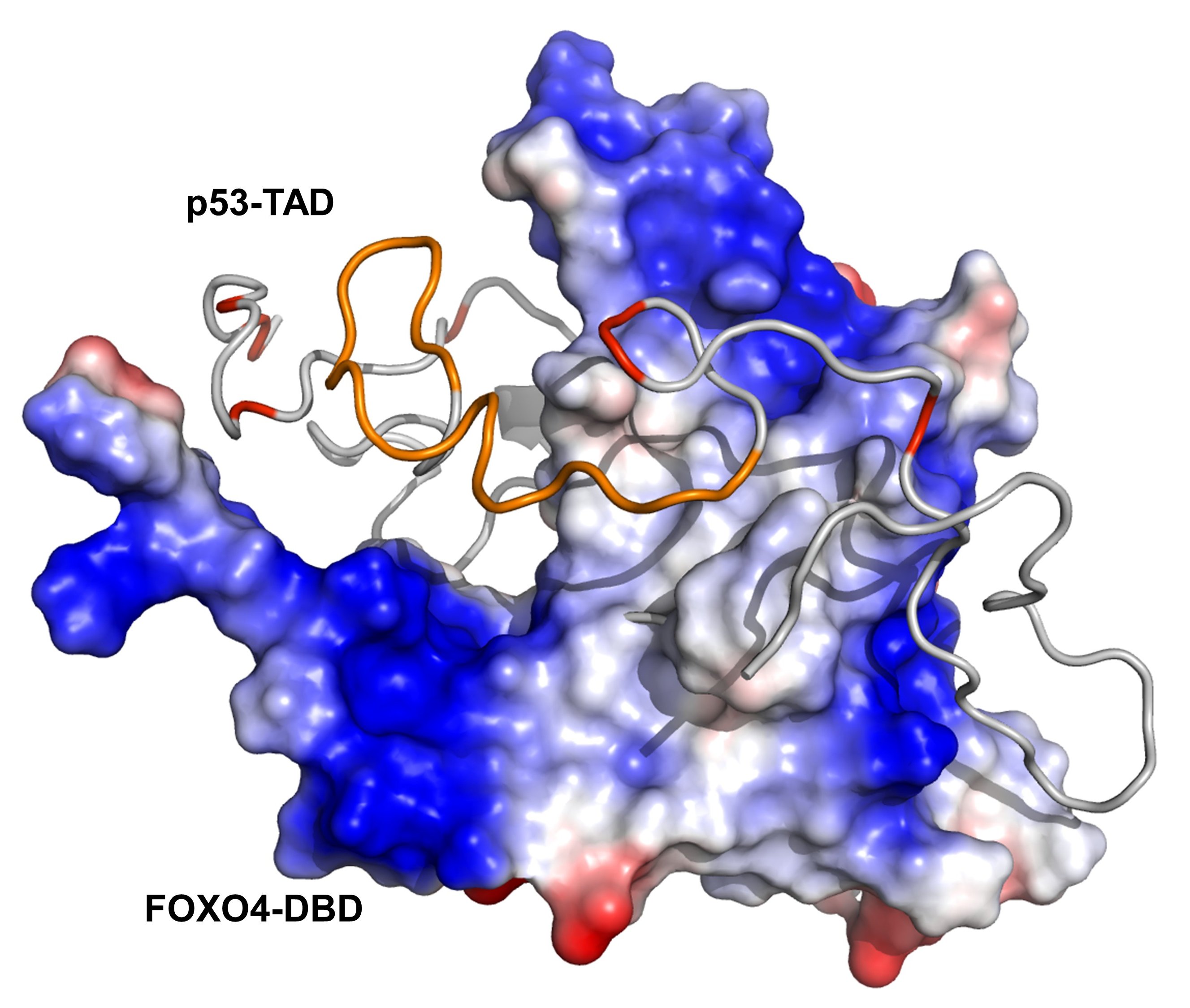

Structure of one of the conformers of the FOXO4-DBD:p53-TAD complex. The surface of FOXO4-DBD is colored according to the electrostatic potential. The p53-TAD protein is shown as a grey ribbon, the TAD2 region is highlighted in orange. Negatively charged Glu and Asp residues of p53-TAD are highlighted in red
27th March 2024
Published a paper in eLife.
Karolina Honzejkova, Dalibor Kosek, Veronika Obsilova* and Tomas Obsil*. The cryo-EM structure of ASK1 reveals an asymmetric architecture allosterically modulated by TRX1. eLife 13:RP95199 (2024). DOI: 10.7554/eLife.95199. IF = 6.4
* shared corresponding authorship
Link: eLife 2024, 13, RP95199
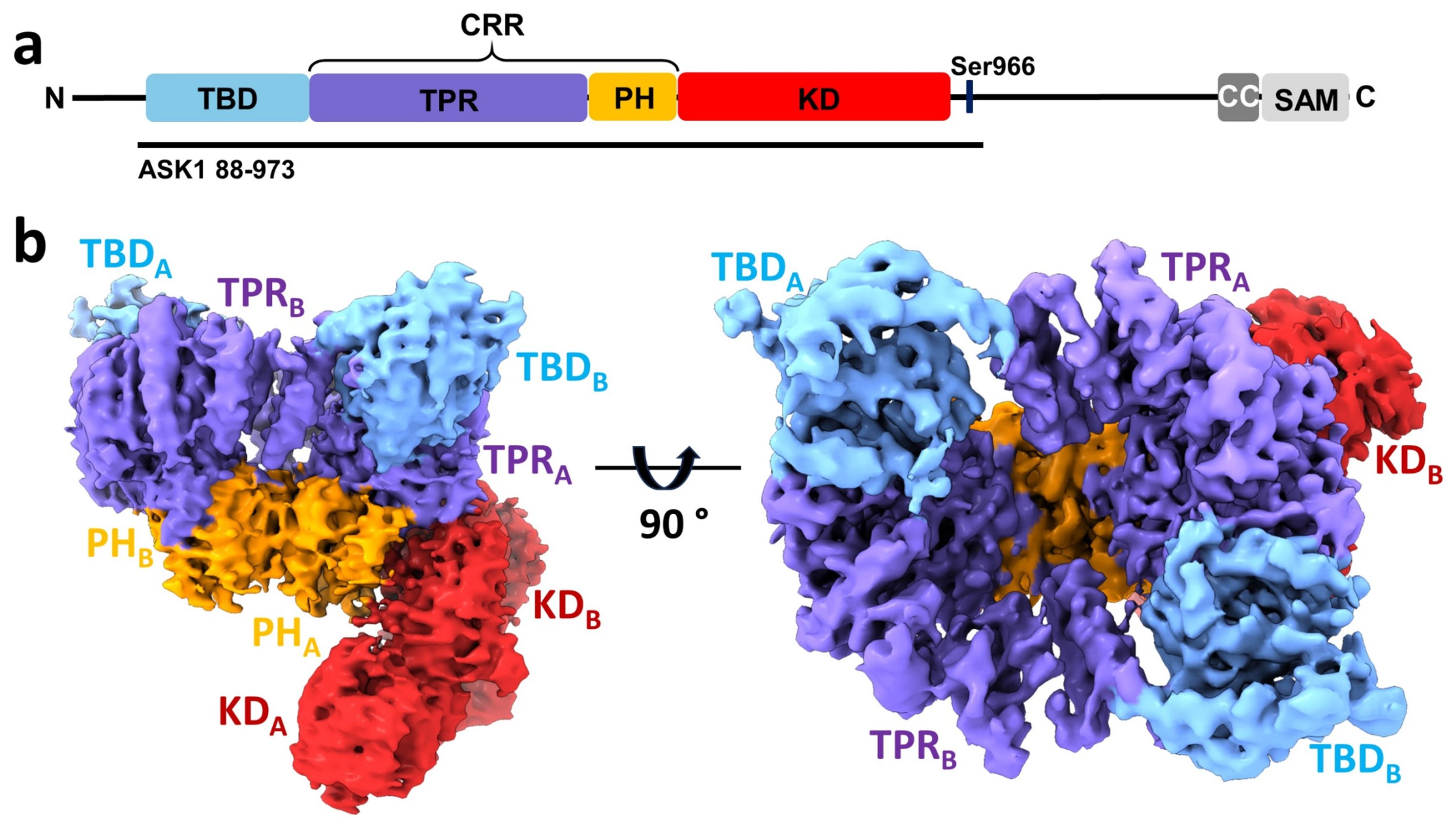
(a) Domain structure of ASK1. TBD, thioredoxin-binding domain; TPR, tetratricopeptide repeat domain; PH, pleckstrin-homology domain; CRR, central regulatory region; KD, kinase domain; CC, coiled-coil motif; SAM, sterile alpha motif domain. (b) Cryo-EM density map of ASK1.