Research
New approaches to in-atomizer trapping of volatile hydrides
In order to decrease the detection limit, the analyte hydride can be easily preconcentrated either in a special collection device (usually by cryogenic trapping) or directly in the atomizer. Cryogenic trapping is time consuming and considerable effort is involved. In contrast, in-atomizer trapping of hydrides is the most convenient way of analyte collection. It can take place on the surface of the atomizer segment that is aligned in the optical path of the instrument. Then the terminology in-situ collection, suggested in one of the first papers on in-atomizer collection in graphite furnaces, accurately reflects the nature of the process. Until recently, the only widely used approach to in-atomizer collection was in-situ collection in graphite furnaces but then procedures based on collection on metal and quartz surfaces emerged.
A compact trap-and-atomizer device based on quartz multiatomizer (Fig.1) has been constructed in our laboratory recently [1]. It was shown to be a powerful tool for determination of hydride forming elements at ultratrace levels by HG-AAS since it allows rapid and efficient in-atomizer preconcentration of the analyte prior its detection by atomic absorption spectrometry. The inlet arm of the multiatomizer has an independent resistive heating and serves as a trap. Two auxiliary channels are employed to introduce oxygen and hydrogen gas streams, respectively, into the trap-and-atomizer device. Oxygen rich atmosphere is used to remove (burn out) hydrogen evolved as a byproduct during the chemical hydride generation of the analyte and thus to reach efficient trapping. On the contrary, hydrogen containing atmosphere is used to volatilize the trapped species.
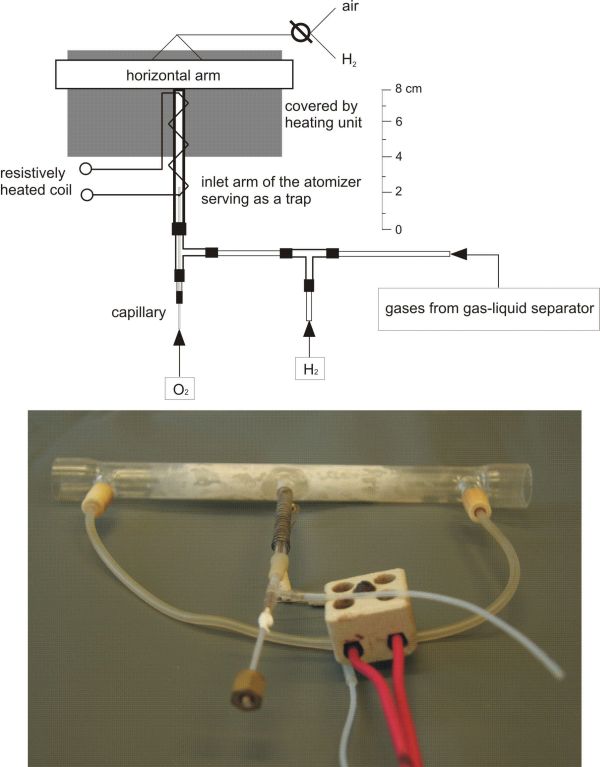
Fig.1 Scheme and photo of a trap-and-atomizer device.
Complete trapping and subsequent volatilization, i.e. 100 % preconcentration efficiency, was reached under the optimized conditions for Sb and Bi [2] as well as Pb [3] and Sn [4]. On the contrary, the overall preconcentration efficiency for As and Se, the most important hydride forming elements, was only between 50-70 % [1]. 100 % preconcentration efficiency was confirmed also by independent radiotracer technique for Sb and Bi [5] as well as Pb [6]. The optimum conditions for trapping of individual elements in a quartz trap-and-atomizer device are summarized in Table 1:
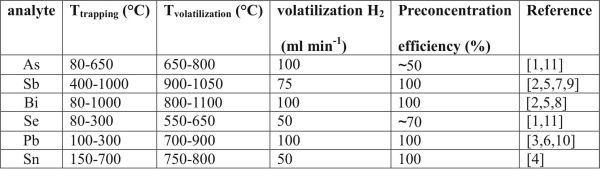
Tab.1 Summary of optimum preconcentration conditions in a trap-and-atomizer device.
The inherent advantages of this novel method and device are compared to the approach used earlier: (i) lower risk of analyte losses during trapping step due to the presence of oxygen excess over hydrogen in the trapping step and (ii) compact design of the device that minimizes temperature gradients between the trap and the optical arm allowing better control of analyte transport losses.
Detection limits in low pg/mL range were reached employing the preconcentration mode (preconcentration period up to 300 s). In-situ trapping in the optical arm of the atomizer might be used instead of in-atomizer trapping (preconcentration in the inlet arm) if trapping, volatilization and atomization temperature values are identical. Under these circumstances, the trapping and volatilization processes are controled only by composition of gaseous phase. As a consequence, both the appratus setup as well as the procedure can be simplified. Such conditions have been found for Sb [7] and Bi [8] owing to the overlap of optimum trapping and volatilization temperature as well as atomization temperature (900 °C) as can be seen from Tab. 1. In-situ collection cannot be reached for As, Se, and Pb since compromise among temperature values for trapping (below 600 °C), volatilization and atomization (900 °C) cannot be reached.
The usefulness of the hydride generation in-atomizer collection for routine praxis was demonstrated on Sb determination in acetic acid leachates from pewter cups, i.e. in complex matrix containing extremely high concentrations of another hydride forming element [9]. Interferences of other hydride forming elements were thoroughly studied for Pb determination employing preconcentration in a trap-and-atomizer device [10] and the results were compared to those without preconcentration. Oxygen used during analyte trapping to improve the trapping efficiency was found to interfere severely on As and Se determination in the preconcentration mode due to its molecular absorption below 200 nm [11].
RELATED PAPERS:
- [1] KRATZER J., DĚDINA J.: Arsine and selenium hydride trapping in a novel quartz device for atomic absorption spectrometry. Anal. Bioanal. Chem. 388 (2007), 793-800.
- [2] KRATZER J., DĚDINA J.: Stibine and bismuthine trapping in quartz tube atomizers for atomic absorption spectrometry - method optimization and analytical applications. Spectrochim. Acta Part B 63 (2008), 843-849.
- [3] KRATZER J.: Ultratrace determination of lead by hydride generation in-atomizer trapping atomic absorption spectrometry: optimization of plumbane generation and analyte preconcentration in a quartz trap-and-atomizer device. Spectrochim. Acta Part B 71-72 (2012) 40-47.
- [4] PRŮŠA L., DĚDINA J., KRATZER J.: Ultratrace determination of tin by hydride generation in-atomizer trapping atomic absorption spectrometry. Anal. Chim. Acta 804 (2013), 50-58.
- [5] KRATZER J., VOBECKÝ, M., DĚDINA J.: Stibine and bismuthine trapping in quartz tube atomizers for atomic absorption spectrometry. Part 2: a radiotracer study. J. Anal. Atom. Spectrom. 24 (2009), 1222-1228.
- [6] KRATZER J., MUSIL S., VOBECKÝ M., DĚDINA J.: Hydride generation - in-atomizer collection of Pb in quartz tube atomizers for atomic absorption spectrometry – a 212Pb radiotracer study. J. Anal. Atom. Spectrom., 28 (2013) 344-353.
- [7] KRATZER J., DĚDINA J.: In situ trapping of stibine in externally heated quartz tube atomizers for atomic absorption spectrometry. Spectrochim. Acta Part B 60 (2005) 859-864.
- [8] KRATZER J., DĚDINA J.: In situ trapping of bismuthine in externally heated quartz tube atomizers for atomic absorption spectrometry. J. Anal. Atom. Spectrom. 21 (2006), 208-210.
- [9] DESSUY M., KRATZER J., GORETI-VALE M., WELZ B., DĚDINA J.: Hydride generation in-atomizer collection atomic absorption spectrometry for the determination of antimony in acetic acid leachates from pewter cups. Talanta, 87 (2011), 255-261.
- [10] NOVOTNÝ P., KRATZER J.: Hydride generation - 1in-atomizer collection of Pb in a quartz trap-and-atomizer device for atomic absorption spectrometry – an interference study. Spectrochim. Acta Part B 79-80 (2013), 77-81.
- [11] KRATZER J., DOČEKAL B., HEITMANN U., DĚDINA J.: Spectral interferences of oxygen and water molecules in hydride generation atomic absorption spectrometry with quartz atomizers: comparison of preconcentration and on-line atomization modes for As and Se determination. J. Anal. Atom. Spectrom., 26 (2011), 2230-2237.


