Research
SPECIATION ANALYSIS OF ARSENIC
Recently, we have reviewed speciation analysis of arsenic based on hydride generation (HG) and coupled with analytical atomic spectrometry [1]. Here, the three basic on-line approaches to speciation analysis of arsenic are outlined: selective HG, generation of methyl substituted arsanes followed by cryotrapping (CT) and postcolumn HG. All these approaches have been followed in our laboratory.
In cooperation with the laboratory of Dr. M. Stýblo at University of North Carolina at Chapel Hill (UNC) we have developed a semi-automated setup for HG-CT analysis [2,3]. The setup, which is built around a flow injection unit, was coupled to AAS with a quartz multiatomizer. Tri- and pentavalent species are distinguished on the basis of selective HG: volatile arsanes from trivalent arsenicals and trimethylarsine oxide are selectively generated from TRIS buffer medium at pH 6, while sums of tri- and pentava
We also applied this analytical system for analysis of arsenic contamination in antimony based drug [9]. Noticeably, we also found that commonly used gas phase dryers based on Nafion membrane cause significant loss of dimethylarsane and complete loss of trimethylarsane, and therefore should be avoided. A NaOH pellet dryer was proposed as a safe alternative for all arsanes [10].
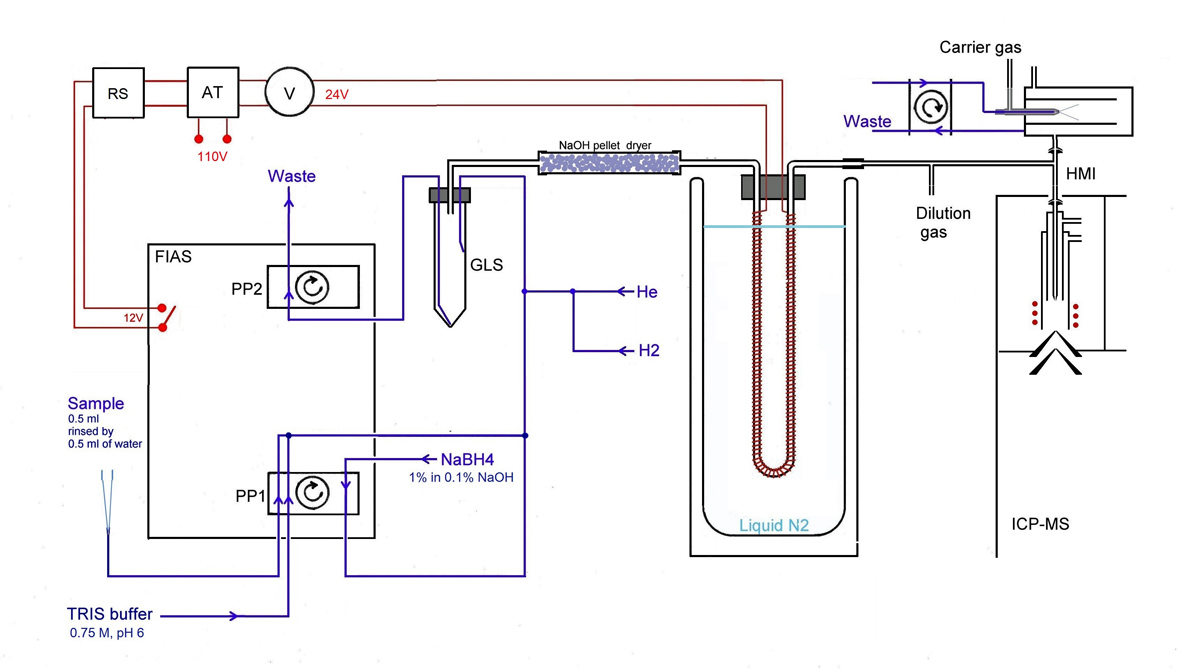
To further improve sensitivity, the HG-CT system was adopted for ICP-MS detection. LODs at or below picogram level allow analysis at trace levels in microsamples [11]. The sensitivity of this method already found application in an in-vitro study finding that arsenic inhibits insuline production in pancreatic cells [12] as well as in a study correlating prevalence of diabetes with concentrations of arsenic species in exfoliated urothelial cells, obtained from arsenic exposed people from Chihuahua region in Mexico [13].
Unique performance of the HG-CT-ICP-MS allowed development and validation of analytical method for speciation analysis of arsenic in full blood even at low exposure levels. Arsenic species are present in blood at ppt levels, much lower concentrations than in urine. Presently, our method is the only one capable of this analysis [14]. Since then, it has been routinely applied to studies of arsenic exposure in association with diabetes at UNC.

Excellent limits of detection of the method qualified us to participate in preparation of Reference Materials AQUA-1 (drinking water), SLRS-6 (river water) and CASS-6 and NASS-7 (sea waters), certified for trace metals, in cooperation with National Research Council Canada. As a sole laboratory, we were able to provide data (information values) for arsenic species in these materials with total arsenic content under 1 µg L-1 [15].
Similar approach was applied also to study of arsenic species in roots and fronds of arsenic hyperaccumulating plants Pteris Cretica in a broad cooperation with University of Chemistry and Technology Prague, Czech University of Life Sciences Prague and Institute of Experimental Botany of the CAS [16].
Excellent limits of detection can be achieved not only with ICP-MS. A connection of HG-CT system to our in-house built atomic fluorescence spectrometer with advanced flame-in-gas-shield atomizer yielded performance comparable to HG-CT-ICP MS but at substantially lower investment and running costs [17].
In the development of HPLC based separation methods, an elegant way around the problem of the nebulizer introduction efficiency is incorporation of a post-column hydride generation step. The effluent from the column is on-line acidified and mixed with the reductant (sodium borohydride solution). Formed gaseous arsenic hydrides are separated from the liquid and introduced to the plasma with essentially 100% efficiency. Previously, we developed an online system for pentavalent arsenic prereduction by thioglycolic (mercaptoacetic) acid, with an efficiency and sensitivity equal to L-cysteine [18]. Alternatively, we developed a postcolumn HG coupled to AFS and achieved equal generation efficiency (100%) of arsanes from arsenite, arsenate, methylarsonate and dimethylarsinate. Two benefits thus emerge: there is just one standard required (e.g. dimethylarsinate) to quantify all four species and time for calibration is substantially shortened because dimethylarsinate typically elutes from the chromatography column at short retention times [19].
We also investigated in detail a serious molecular rearrangement (demethylation) of arsenic species during HG from HCl, which is the most common generation medium, and refuted the general assumption that only corresponding arsanes are volatile products from methylated As species [20]. This behavior can jeopardize accuracy of As speciation analysis based on generation of methyl-substituted hydrides which is occasionally used for toxicological studies. We identified the hydrolytic products of NaBH4 responsible for demethylation. In a following paper [21], we tried to make use of this behavior, namely, that under certain conditions of HG the bond between As and C can be broken. We investigated HG activity of complex As species, i.e., arsenosugars, with a focus on reaction mechanism. The highest yield of volatile hydrides from arsenosugars was obtained from sulfuric acid medium. With the knowledge of the mechanism we have improved the design of the generator to enhance the generation efficiency and applied this design to test feasibility of postcolumn HG for determination of arsenosugars.

One of main fields of analytical applications is the safety of food. Arsenic is found at elevated levels especially in rice and seafood. However, arsenic is present in species of very different toxicity. In fact, in seafood majority of arsenic is found in the form of non-toxic species, mainly arsenobetaine. According to reaction conditions, such as pH of reaction mixture, hydrides of arsenic can be selectively generated only from species of interest. This can be used for fast screening of presence of toxic and carcinogenic inorganic arsenic (iAs) in food, much faster and less expensive than complete speciation analysis by HPLC-ICP-MS [22, 23, 24], a basis to an Agilent Application Note [25] (in cooperation with prof. J. Feldmann from TESLA laboratory, University of Aberdeen).
Although our main focus is on arsenic speciation analysis in biological materials, we are open to collaboration in other areas. For instance, we analyzed iAs(III) and iAs(V) by HPLC-ICP-MS in samples of water for a study of arsenic mobilization processes in a natural As geochemical anomaly and in soils (in cooperation with Institute of Geochemistry, Mineralogy and Mineral Resources, Faculty of Sciences, Charles University in Prague) [26-29].
RELATED PAPERS:
- [1] MUSIL S.: Speciation Analysis of Arsenic Based on Hydride Generation (Speciační analýza arsenu založená na generování hydridů). Chemické listy, 114 (2020), 374–381.
- [2] MATOUŠEK T., HERNANDÉZ-ZAVALA A., SVOBODA M., LANGEROVÁ L., ADAIR B. M., DROBNÁ Z., THOMAS D. J., STÝBLO M., DĚDINA J.: Oxidation State Specific Generation of Arsines from Methylated Arsenicals Based on L-Cysteine Treatment in Buffered Media for Speciation Analysis by Hydride Generation-Automated Cryotrapping-Gas Chromatography-Atomic Absorption Spectrometry with the Multiatomizer. Spectrochimica Acta Part B 63 (2008) 396–406.
- [3] HERNANDEZ-ZAVALA A., MATOUSEK T., DROBNA Z., PAUL D.S., WALTON F.S., ADAIR B.M., DEDINA J., THOMAS D.J. AND STYBLO M.: Speciation Analysis of Arsenic in Biological Matrices by Automated Hydride Generation-Cryotrapping-Atomic Absorption Spectrometry with Multiple Microflame Quartz Tube Atomizer (Multiatomizer). Journal of Analytical Atomic Spectrometry, 23 (2008), 342-351.
- [4] D. S. PAUL, A. HERNANDEZ-ZAVALA, F.S. WALTON, B.M. ADAIR, J.DĚDINA, T. MATOUŠEK and M. STÝBLO: Examination of the effects of arsenic on glucose homeostasis in cell culture and animal studies: Development of a mouse model for arsenic-induced diabetes. Toxicology and Applied Pharmacology, 222 (2007) 305-314.
- [5] HERNANDEZ-ZAVALA A., VALENZUELA O.L.. MATOUŠEK T., DROBNÁ Z., DĚDINA J., GARCIA-VARGAS G.G., THOMAS D.J., DEL RAZO L.M., STÝBLO M.: Speciation of arsenic in exfoliated urinary bladder epithelia cells from individuals exposed to arsenic in drinking water. Environmental Health Perspectives 116 (2008), 1656-1660.
- [6] CURRIER J., SVOBODA M., DE MORAES D., MATOUŠEK T., DĚDINA J., STÝBLO M.: Direct Analysis of Methylated Trivalent Arsenicals in Mouse Liver by Hydride Generation-Cryotrapping-Atomic Absorption Spectrometry. Chemical Research in Toxicology, 24 (2011), 478-480.
- [7] CURRIER J.M., SVOBODA M., MATOUŠEK T., DĚDINA J., STÝBLO M.: Direct Analysis and Stability of Methylated Trivalent Arsenic Metabolites in Cells and Tissues. Metallomics, 3 (2011), 1347-1354.
- [8] CURRIER J., SAUNDERS J., DING L., BODNAR W., CABLE P., MATOUŠEK T., CREED J., STÝBLO M.: Comparative oxidation state specific analysis of arsenic species by high-performance liquid chromatography-inductively coupled plasma-mass spectrometry and hydride generation-cryotrapping-atomic absorption spectrometry. Journal of Analytical Atomic Spectrometry, 28 (2013), 843-852.
- [9] MORAES D.P., SVOBODA M., MATOUŠEK T., FLORES E.M.M., DĚDINA J.: Selective Generation of Substituted Arsines-Cryotrapping-Atomic Absorption Spectrometry for Arsenic Speciation Analysis in N-methylglucamine antimonate. Journal of Analytical Atomic Spectrometry, 27 (2012), 1734-1742.
- [10] TAURKOVÁ P., SVOBODA M., MUSIL S., MATOUŠEK T.: Loss of di- and trimethylarsine on Nafion membrane dryers following hydride generation. Journal of Analytical Atomic Spectrometry, 26 (2011), 220-223.
- [11] MATOUŠEK T., CURRIER J.M. TROJÁNKOVÁ N., SAUNDERS J.R., ISHIDA M.C., GONZALEZ-HORTA C., MUSIL S., MESTER Z, STÝBLO M, DĚDINA J.: Selective hydride generation – cryotrapping – ICP-MS for arsenic speciation analysis at picogram levels: analysis of river and sea water reference materials and human bladder epithelial cells. Journal of Analytical Atomic Spectrometry, 28 (2013), 1456 – 1465.
- [12] DOUILLET C., CURRIER J., SAUNDERS J., BODNAR W. M., MATOUSEK T., STYBLO M.: Methylated trivalent arsenicals are potent inhibitors of glucose stimulated insulin secretion by murine pancreatic islets. Toxicology and Applied Pharmacology, 267 (2013) 11-15.
- [13] CURRIER J. M., ISHIDA M. C., GONZÁLEZ-HORTA C., SÁNCHEZ-RAMÍREZ B., BALLINAS-CASARRUBIAS L., GUTIÉRREZ-TORRES D. S., CERÓN R. H., MORALES D. V., TERRAZAS, F. A. B., DEL RAZO L. M., GARCÍA-VARGAS G. G., SAUNDERS, R. J., DROBNÁ, Z., FRY, R. C., MATOUŠEK T., MENDEZ M. A., LOOMIS D, STÝBLO, M.: Associations between arsenic species in exfoliated urothelial cells and prevalence of diabetes among residents of Chihuahua, Mexico. Environmental Health Perspectives 122 (2014), 1088-1094.
- [14] MATOUŠEK T., WANG Z. F., DOUILLET C., MUSIL S., STÝBLO M.: Direct Speciation Analysis of Arsenic in Whole Blood and Blood Plasma at Low Exposure Levels by Hydride Generation-Cryotrapping-Inductively Coupled Plasma Mass Spectrometry Analytical Chemistry, Analytical Chemistry, 89 (2017), 9633–9637.
- [15] YANG L., NADEAU K., MEIJA J., GRINBERG P., PAGLIANO E., ARDINI F., GROTTI M., SCHLOSSER CH., STREU P., ACHTERBERG E.P., SOHRIN Y., MINAMI T., ZHENG L., WU J., CHEN G., ELLWOOD M.J., TURETTA C., AGUILAR-ISLAS A., REMBER R., SARTHOU G., TONNARD M., PLANQUETTE H., MATOUŠEK T., CRUM S., MESTER Z.: Inter-laboratory study for the certification of trace elements in seawater certified reference materials NASS-7 and CASS-6. Analytical and Bioanalytical Chemistry 410 (2018), 4469-4479.
- [16] POPOV M., ZEMANOVÁ V., SÁCKÝ J., PAVLÍK M., LEONHARDT T., MATOUŠEK T., KAŇA A., PAVLÍKOVÁ D., KOTRBA P.:Arsenic accumulation and speciation in two cultivars of Pteris cretica L. and characterization of arsenate reductase PcACR2 and arsenite transporter PcACR3 genes in hyperaccumulating cv. Albo-lineata, Ecotoxicology and Environmental Safety (2020), submitted.
- [17] MUSIL S., MATOUŠEK T., CURRIER J. M., STÝBLO M., DĚDINA J.: Speciation Analysis of Arsenic by Selective Hydride Generation-Cryotrapping-Atomic Fluorescence Spectrometry with Flame-in-Gas-Shield Atomizer: Achieving Extremely Low Detection Limits with Inexpensive Instrumentation. Analytical Chemistry, 86 (2014), 10422-10428.
- [18] MUSIL S., MATOUŠEK T.: On-line pre-reduction of pentavalent arsenicals by thioglycolic acid for speciation analysis by selective hydride generation-cryotrapping-atomic absorption spectrometry. Spectrochimica Acta Part B: Atomic Spectroscopy 63 (2008) 685-691.
- [19] MARSCHNER K., MUSIL S., DĚDINA J.: Achieving 100% Efficient Postcolumn Hydride Generation for As Speciation Analysis by Atomic Fluorescence Spectrometry. Analytical Chemistry 88 (2016) 4041−4047,
- [20] MARSCHNER K., MUSIL S., DĚDINA J.: Demethylation of Methylated Arsenic Species during Generation of Arsanes with Tetrahydridoborate(1−) in Acidic Media. Analytical Chemistry 88 (2016) 6366−6373, DOI:10.1021/acs.analchem.6b00735
- [21] MARSCHNER K., MUSIL S., MIKŠÍK I., DĚDINA J.: Investigation of hydride generation from arsenosugars - Is it feasible for speciation analysis? Analytica Chimica Acta, 1008 (2018), 8-17.
- [22] MUSIL S., PÉTURSDÓTTIR Á. H., RAAB A., GUNNLAUGSDÓTTIR H., KRUPP E., FELDMANN J.: Speciation without Chromatography Using Selective Hydride Generation: Inorganic Arsenic in Rice and Samples of Marine Origin. Analytical Chemistry, 86 (2014), 993–999.
- [23] PÉTURSDÓTTIR Á. H., FRIEDRICH N., MUSIL S., RAAB A., GUNNLAUGSDÓTTIR H., KRUPP E. M., FELDMANN J.: Hydride generation ICP-MS as a simple method for determination of inorganic arsenic in rice for routine biomonitoring. Analytical Methods, 6 (2014), 5392–5396.
- [24] MARSCHNER K., PÉTURSDÓTTIR Á. H., BÜCKER P., RAAB A., FELDMANN J., MESTER Z., MATOUŠEK T., MUSIL S.: Validation and inter-laboratory study of selective hydride generation for fast screening of inorganic arsenic in seafood. Analytica Chimica Acta, 1049 (2019), 20–28.
- [25] PÉTURSDÓTTIR Á. H., MUSIL S., FRIEDRICH N., KRUPP E. M., FELDMANN J, GUNNLAUGSDÓTTIR H.: High Throughput Determination of Inorganic Arsenic in Rice using Hydride Generation-ICP-MS, Application Note in Handboook of ICP-QQQ applications using the Agilent 8800 and 8900, Agilent Application Note 5991-6055EN (2015); https://www.agilent.com/cs/library/applications/5991-6055EN_AppNote_8800_ICP-MS_As_rice_hydride.pdf
- [26] DRAHOTA P., FALTEISEK L., REDLICH A., ROHOVEC J., MATOUŠEK T., ČEPIČKA I.: Microbial effects on the release and attenuation of arsenic in the shallow subsurface of a natural geochemical anomaly. Environmental Pollution, 180 (2013), 84-91.
- [27] DRAHOTA P., NOVÁKOVÁ B., MATOUŠEK T, MIHALJEVIČ M., ROHOVEC J., FILIPPI M.: Diel variation of arsenic, molybdenum and antimony in a stream draining natural As geochemical anomaly. Applied Geochemistry 31 (2013), 84-93.
- [28] JAROŠÍKOVÁ, A., ETTLER, V., MIHALJEVIČ, M., PENÍŽEK, V., MATOUŠEK, T., CULKA, A., DRAHOTA, P.: Transformation of arsenic-rich copper smelter flue dust in contrasting soils: A 2-year field experiment. Environmental Pollution. 237 (2018), 83-92.
- [29] KNAPPOVÁ M., DRAHOTA P., FALTEISEK L., CULKA A.., PENÍŽEK V., TRUBAČ, J., MIHALJEVIČ M., MATOUŠEK T.: Microbial sulfidogenesis of arsenic in naturally contaminated wetland soil. Geochimica et Cosmochimica Acta. 267 (2019), 33-50.
PATENTS:
- MATOUŠEK T.: Unit for cryotrapping of volatile compounds. Czech patent no. 302885, issued by Industrial Property Office of the Czech Republic (23.11. 2011).


